Science and Technology Frontier | Scanning of Recent Scientific Research Achievements
Team Xiang Ye of Medical College made a breakthrough in the research of African swine fever virus.
Li Pilong’s research group of School of Life and Li Haitao’s research group of Medical College cooperated to report the new mechanism of histone modification regulating chromatin compartmentalization by promoting phase separation.
The Engineering Laboratory of Medical College reported the key factors regulating the spread of dengue virus and put forward a new anti-dengue blocking strategy.
The research group of Li Yue, Department of Electronics, issued a report on the realization of near-zero exponential concept devices by dielectric integrated optical doping.
Fang Xianyang’s research group of School of Life has made new progress in the solution structure study of long-chain non-coding subgenomic RNA of flavivirus.
Zhu Junming and Xue Lan of the School of Public Administration jointly published a document to reveal the impact of China’s carbon emission trading policy on low-carbon innovation.
Zhong Yi’s research group of School of Life reveals the downstream molecular mechanism of active forgetting of different memory components.
Xiao Bailong from the School of Pharmacy and Li Xueming from the School of Life jointly published a paper in Nature.
Reveal the delicate molecular machines structure and mechanism that mediates human tactile perception.

Team Xiang Ye of Tsinghua Medical College has made a breakthrough in the research of African swine fever virus.
On September 17th, a team led by Xiang Ye, an associate professor hired by Tsinghua University Medical Dean and a researcher at Beijing Advanced Innovation Center for Structural Biology, published an academic paper entitled "Structure of the African Swine fever virus major capsid protein p72" online in Cell Research. In this study, the high-resolution structure of African classical swine fever virus capsid protein p72 was analyzed for the first time by the method of three-dimensional reconstruction of single particle by cryoelectron microscope, and the possible assembly mechanism of African classical swine fever virus capsid was revealed, which laid a solid foundation for the development of African classical swine fever virus subunit vaccine.
African swine fever virus originated in Africa, and the mortality rate of domestic pig infection is nearly 100%, which is a great threat to domestic pig breeding industry and even people’s livelihood and economy. African swine fever virus has spread rapidly from Africa to Europe, South America and some parts of Asia in the past few decades. In August 2018, African swine fever virus infection was found in northeast China, and soon the virus spread to most parts of the country, causing economic losses of more than 100 billion yuan. At present, it is urgent to develop effective vaccines and epidemic prevention measures to control and prevent African swine fever virus. In response to the urgent needs of the country, Tsinghua University Beijing Advanced Innovation Center for Structural Biology and Beijing Frontier Research Center for Biological Structure took the lead, and more than a dozen research groups from Peking University, Harbin Veterinary Research Institute of Chinese Academy of Agricultural Sciences, Shanghai Pasteur Institute and other units began to tackle key problems of African swine fever virus in April this year.
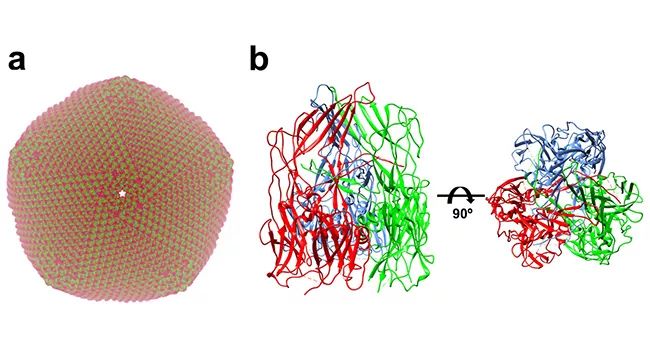
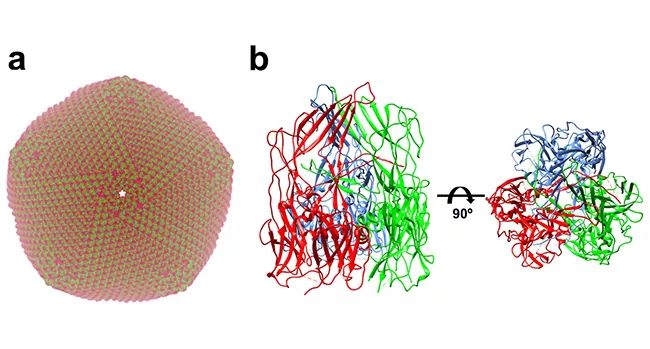
▲ Structure of African classical swine fever virus: a. Simulate the capsid structure of African classical swine fever virus produced by virus assembly according to the capsid protein p72 structure of African classical swine fever virus; B freeze electron microscopic structure of African classical swine fever virus capsid protein p72 trimer.
African swine fever virus is a double-stranded DNA virus with complex structure. Its genome is protected by two capsids and two outer membranes. The capsids are mainly composed of virus coding protein p72. According to previous experimental data, p72 accounts for about 33% of the total weight of virus particles, and p72 is the main antigen that can be detected in the blood of infected pigs. At the initial stage of studying p72 protein, Xiang Ye’s research group found that p72 protein with correct folding conformation could not be obtained by recombinant expression of p72 protein alone, and only when p72 was co-expressed with another helper protein B602L encoded by African swine fever, could the p72 protein with correct folding conformation be obtained. On this premise, the research team determined the high-resolution 3-D structure of p72 protein of 2.67 angstrom (above) by using the method of single particle 3-D reconstruction with cryoelectron microscope. The results showed that three p72 molecules formed a stable trimer form. Each p72 contains two pancake-like domains connected in series, and each domain consists of eight β-folded sheets, which is very common in the capsid protein structure of icosahedral virus. The six cake-like structures of trimer p72 together form a pseudo-hexamer whose bottom is anchored on the inner membrane of the virus. The inserted fragments between trimer p72 folded sheets together form a propeller-like top structure that extends out of the virus, which is most likely the receptor binding area on the cell surface.
There have been many attempts to study subunit vaccines based on p72 protein of African classical swine fever virus, but no satisfactory results have been obtained. The failure of previous studies is probably due to the failure to use the correctly folded p72 protein. Obtaining the correctly folded p72 protein and determining its high-resolution structure are helpful to develop drugs targeting virus capsid assembly and subunit vaccines. At present, the research and development and evaluation of p72 subunit vaccine are being closely carried out by Tsinghua University and Harbin Veterinary Research Institute of Chinese Academy of Agricultural Sciences.
Associate Professor Xiang Ye is the correspondent of this article. Liu Qi, a 2014 Ph.D. student from Tsinghua University Life Union Center, is the first author of this paper. Ma Bingting, a 2015 Ph.D. student from Xiangye Laboratory, Associate Professor Tan Xu from Tsinghua University College of Pharmacy and their laboratory members Qian Nianchao and Zhang Fan, and Professor Lei Jianlin, director of the cryo-electron microscope platform of Tsinghua University Life College, participated in this work.
Paper link:
https://www.nature.com/articles/s41422-019-0232-x

Li Pilong’s research group of Tsinghua Life College cooperated with Li Haitao’s research group of Medical College to report the new mechanism of histone modification regulating chromatin compartmentalization by promoting phase separation.
On September 16th, Li Pilong’s research group of Tsinghua University Life College cooperated with Li Haitao’s research group of Tsinghua University Medical College. An academic article entitled "Histone modifications regulate chromatin compartmentalization by contributing to a phase separation mechanism" was published in Molecular Cell.
Eukaryotic cells use many membrane-bound organelles to distinguish biochemical reactions in order to better regulate space and time. For example, their genomes are enclosed in a membrane-bound organelle, namely the nucleus, in order to better control the genetic information flow. In the nucleus, the genome is further regulated to form many compartments or regions, which play an important role in regulating gene expression. Judging from some of their characteristics, these dynamic solid compartments are similar to membraneless organelles. Many protein that play a role in chromatin compartmentalization have the inherent ability of liquid-liquid separation, such as human heterochromatin protein HP1α and Drosophila heterochromatin protein HP1a. Based on these observations, it has been suggested that chromatin compartmentalization is formed by liquid-liquid separation. However, the previous studies can’t explain why the other two homologous proteins, HP1β and HP1γ, of human heterochromatin protein HP1α, which can’t be separated by themselves or from DNA, perform similar functions, and whether the formation of constitutive heterochromatin is regulated by the liquid-liquid separation mechanism formed by multivalent interaction between multivalent HP1 protein complex and multivalent histone H3K9 trimethyl (H3K9me3).
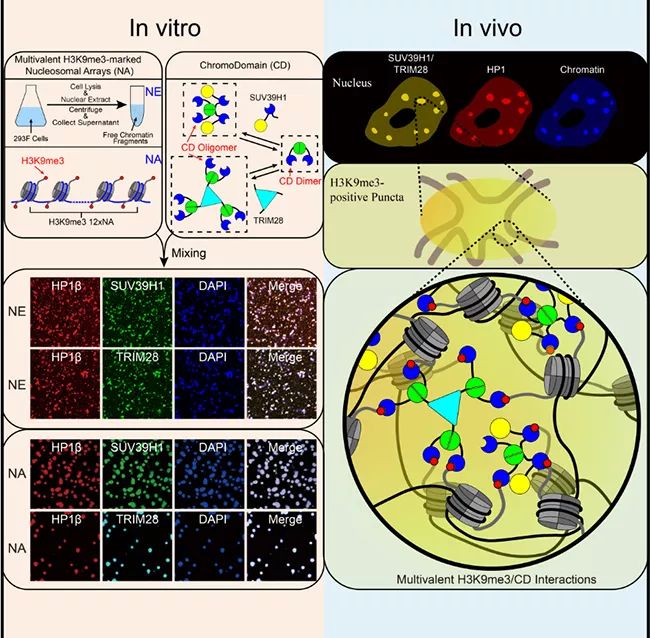
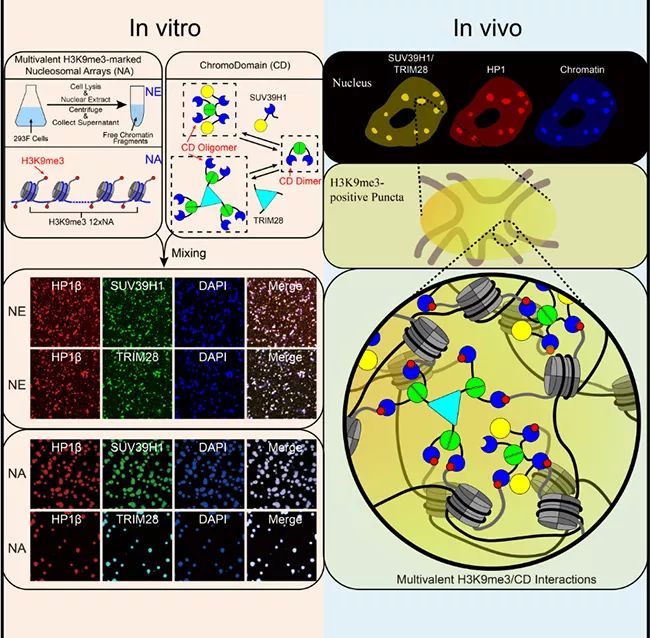
▲ Liquid-liquid separation mediated by multivalent interaction between H3K9me3 on nucleosome string and CD of HP1 complex promotes the formation of heterochromatin compartments.
In this work, Li Pilong and Li Haitao’s research group cooperated to prove that in vitro, the modification of histone H3K9 trimethyl (H3K9me3) and the multivalent interaction of recognizing its modified HP1 chromatin domain Chromodomain(CD) can lead to the formation of heterochromatin. The authors used two polyvalent HP1-CD complexes, the key chromatin fragment (NE) extracted from human nucleus and the nucleosome string (NA) modified by H3K9me3 in vitro as experimental systems. Several evidences show that the formation of these droplets is mediated by liquid-liquid separation. The resulting droplets are reminiscent of the dynamic behavior of heterochromatin and its response to biochemical regulation and mutant phenotype. The author concludes that the liquid-liquid separation driven by multivalent H3K9me3/CD interaction is the key force for the formation of heterochromatin in cells. To sum up, this study proposed a general mechanism of histone labeling regulating chromosome compartmentalization by promoting liquid-liquid separation.
Li Pilong and Li Haitao are co-authors of this article. Wang Liang, a 2015 Ph.D. student from Tsinghua University Life College, Gao Yifei, a 2016 Ph.D. student from Medical College, and Zheng Xiangdong, a postdoctoral fellow from Medical College, are the co-first authors of this article.
Paper link:
https://www.cell.com/molecular-cell/fulltext/S1097-2765(19)30658-6

Cheng Gong Laboratory of Tsinghua Medical College reported the key factors regulating the spread of dengue virus and proposed a new anti-dengue blocking strategy.
On September 16th, Professor Cheng Gong from Tsinghua University Medical College published an academic paper entitled "Host serum iron modulations of dengue virus acquisition by mosquitoes" (doi: 10.1038/S41564-019-0555-) in Nature Microbiology. This study found for the first time that the iron content in human serum is the key factor to regulate the transmission of dengue virus by mosquitoes, and put forward an anti-dengue transmission blocking strategy based on iron supplementation.
Dengue fever is one of the most widespread viral infectious diseases in the world. Dengue virus is carried by mosquitoes and spread to people. At present, dengue fever has been prevalent in more than 100 countries and regions around the world. According to the estimation of the World Health Organization, about 2.5 billion people in the world live in areas threatened by dengue infection, 390 million people are infected or repeatedly infected with dengue virus every year, and 500-1 million people are hospitalized. Dengue fever has become the largest insect-borne viral infectious disease in the world. In recent 20 years, the epidemic trend of dengue fever in the world has intensified, which has become a worldwide public health problem. Aedes aegypti and Aedes albopictus are the primary vectors of dengue virus transmission in nature. This kind of mosquitoes are widely distributed in tropical and subtropical regions, but the epidemic trend of dengue fever is not completely consistent with the distribution of mosquitoes, and there are obvious population and regional differences in dengue fever. Identifying the key factors affecting the spread of dengue virus is a key scientific problem to be answered in this field.
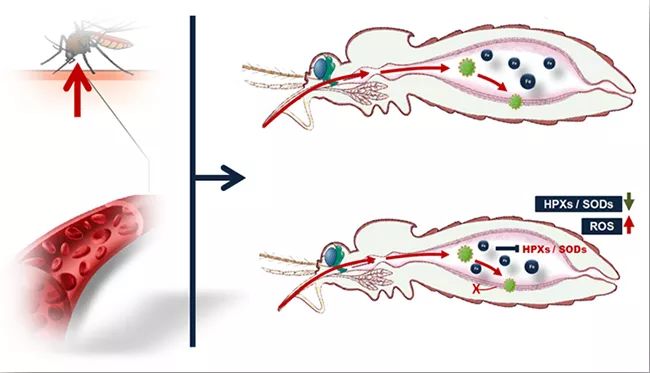
Mosquitoes can acquire viruses from infected hosts by sucking blood. Within a few days after mosquitoes suck blood, the infected person’s blood is quickly digested. At the same time, the virus in the blood infects the intestinal epithelial cells of mosquitoes and spreads into the body cavity of mosquitoes, so that mosquitoes have the ability to spread viruses. The above two processes are highly coincident in time, so researchers speculate that host blood factors or blood metabolites can regulate the process of virus infection in mosquitoes, and ultimately determine the vector efficiency of mosquitoes carrying and spreading viruses. Through a series of screening, it was found for the first time that the iron ion content in the host serum was highly negatively correlated with the ability of mosquitoes to acquire virus by blood-sucking, and it was proved that the iron ion concentration in the serum was the key negative regulatory factor to inhibit mosquitoes from acquiring virus infection. The results of further research show that iron ions in serum can be directly utilized by the iron metabolism system of intestinal cells after entering the intestinal tract of mosquitoes, which inhibits the expression of antioxidant enzyme genes in cells, leading to a significant increase in the level of reactive oxygen species (ROS) in the intestinal tract of mosquitoes, and finally inhibits the infection and amplification of viruses in mosquitoes (Figure 1).
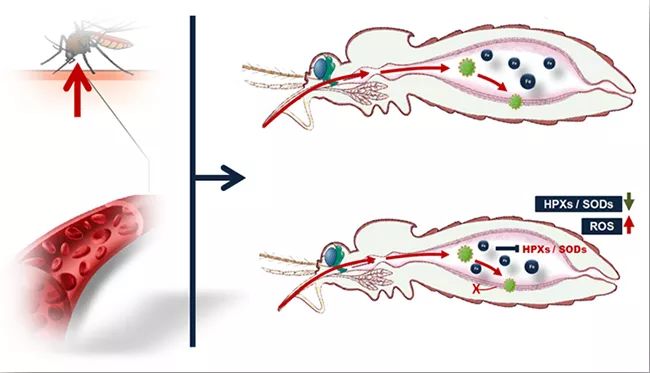
▲ Figure 1. Host serum iron ions can increase the level of reactive oxygen species in the intestinal tract of mosquitoes and inhibit the infection of dengue virus in mosquitoes by inhibiting the expression of antioxidant enzyme genes in intestinal cells.
Iron deficiency is a nutritional problem widely existing in nature. According to the World Health Organization, iron deficiency is widely distributed in Africa, South America, Southeast Asia and other regions. By comparison, it can be found that the distribution area of iron deficiency is highly coincident with the epidemic area of dengue fever, so the researchers speculate that the iron deficiency state of the population is closely related to the epidemic of dengue virus. In the subsequent study, it was found that the low iron state of the host can promote mosquitoes to infect the virus during blood feeding, and iron supplementation to the low iron host can block the spread of the virus. It shows that universal iron supplementation is an effective means to cut off the spread of dengue virus in nature, which will provide a new prevention and control idea and strategy for dengue virus prevention and control.
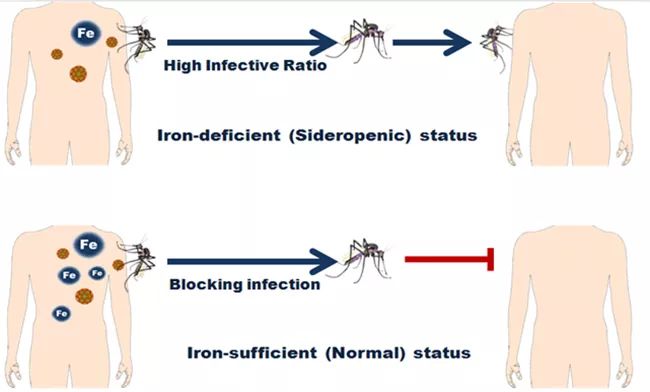
▲ Figure 2. Iron supplementation to the host can block mosquitoes from carrying and spreading dengue virus.
Professor Cheng Gong of Tsinghua University Medical College is the correspondent of this paper, and Zhu Yibin, a postdoctoral fellow of Tsinghua University Medical College, is the first author. Professor Wang Penghua from the Medical College of the University of Connecticut, Professor Liu Qiyong from the Institute of Infectious Diseases of the Chinese Center for Disease Control and Prevention, Professor Bian Zhongqi from the Department of Infectious Diseases of the 920th Hospital of China People’s Liberation Army Joint Logistics Support Force, Professor Itsanun Wiwatanaratanabutr from Thailand Xianhuang Institute of Technology, and Wu Tianshi, deputy chief physician of Tsinghua University School Hospital, are the collaborators of this paper.
Paper link:
https://www.nature.com/articles/s41564-019-0555-x

The research group of Li Yue, Department of Electronics, Tsinghua, published a report in Nature Communication on the realization of near-zero exponential concept devices through dielectric integrated optical doping.
On September 11th, the research group of Associate Professor Li Yue of Tsinghua University Department of Electronic Engineering published a research paper entitled "Substance-integrated photonic doping for near-zero-index devices" in Nature Communications. It is the first time to propose an optical doping method that can be integrated with dielectric to realize functional devices with near-zero exponential electromagnetic characteristics, which provides a new design method for new chip integrated circuits and has potential application value in biosensor, integrated circuits, functional materials and other fields.
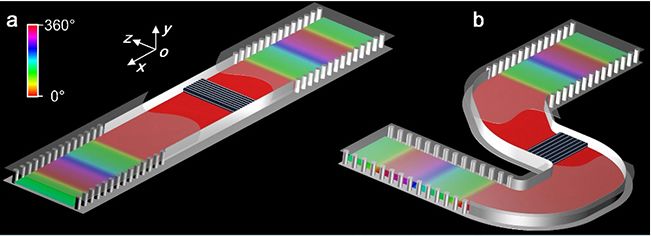
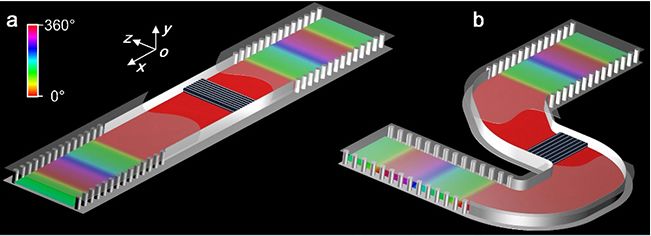
▲ Conceptual diagram of dielectric integrated optical doping: (a) linear structure, (b) bending structure.
Near-zero-index device is a kind of functional device based on near-zero index medium. Compared with traditional electromagnetic devices, it has the characteristics of deformation, local adjustment and field enhancement. Electromagnetic wave in near-zero exponential medium presents a special wave effect of spatial static distribution, including near-zero refractive index and group velocity, as well as infinite wavelength and phase velocity. In 2017, Professor Nader Engheta and Associate Professor Li Yue of the Department of Electrical and Systems Engineering of the University of Pennsylvania jointly published an article in the journal Science, proposing the concept of optical doping based on near-zero exponential media for the first time, transplanting doping technology from micro-scale to macro-scale, and regulating the magnetic permeability of equivalent media through doping in non-ordered media, thus realizing multi-modal control such as paramagnetic, diamagnetic and ideal magnet. It is a kind of aperiodic structure.
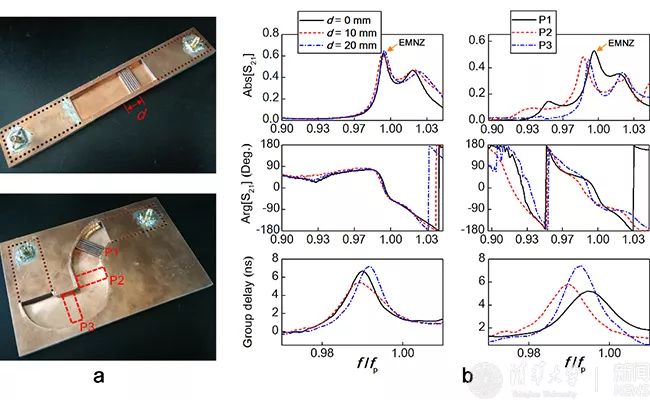
▲ Experimental observation of optical doping characteristics of dielectric integration: (a) test platform, (b) near-zero reflection, zero phase and high group delay.
Based on the engineering application of the new theory, this paper puts forward an optical doping method based on dielectric integration. Combining with typical integrated circuit technology, the regulation of optical doping on the magnetic characteristics of equivalent media is expressed as the regulation of the electromagnetic response of chip integrated devices, and a variety of near-zero exponential functional devices are realized. For example, a high-sensitivity biosensor with the help of local field enhancement effect in doped media, an efficient photoacoustic modulator with weak mechanical vibration, and an "electric fiber" (analogous to optical fiber) that can be bent and deformed at will and its working frequency remains unchanged. In addition, Li Yue team verified the electromagnetic characteristics of the concept of dielectric integrated optical doping through experiments, and observed zero phase shift, high time delay, position independence of doped media and device shape independence, which were consistent with the analytical theoretical prediction and full-wave simulation results.
The theoretical and experimental work of this thesis was completed in Tsinghua University, and the Department of Electronic Engineering of Tsinghua University was the first unit of this thesis. Li Yue and Nader Enkhtar are the corresponding authors of this article, and Zhou Ziheng, a doctoral student in the Department of Electronic Engineering, is the first author. Other authors include Li Hao and Sun Wangyu, Ph.D. students from Tsinghua University Department of Electronic Engineering, and Dr. Inigo Liberal from Navarre Public University.
Paper link:
https://www.nature.com/articles/s41467-019-12083-y

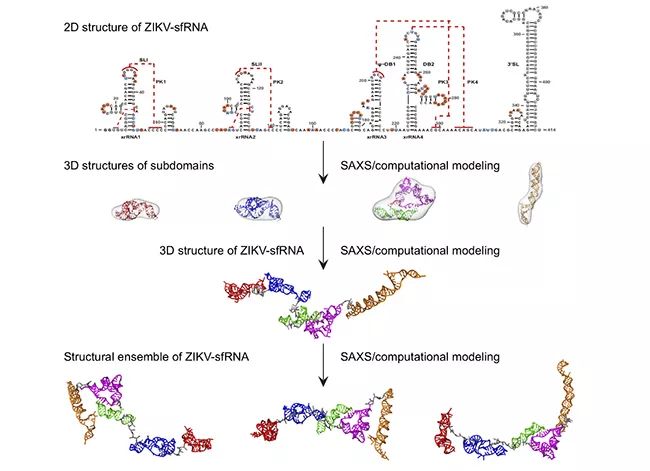
Fang Xianyang’s research group of Tsinghua Institute of Life has made new progress in the study of solution structure of long-chain non-coding subgenomic RNA of flavivirus.
On September 10th, Fang Xianyang’s research group of the School of Life cooperated with Qin Chengfeng’s research group of the Institute of Microbiology and Epidemiology of the Academy of Military Medicine. A research paper entitled "Long non-coding subgenomic RNA of flavivirus has extended 3D structures and is flexible in solution" was published in EMBO Reports. In this paper, the solution structure of long-chain non-coding subgenomic RNA from flavivirus type 2 dengue virus, Zika virus and West Nile virus was reported in detail for the first time.
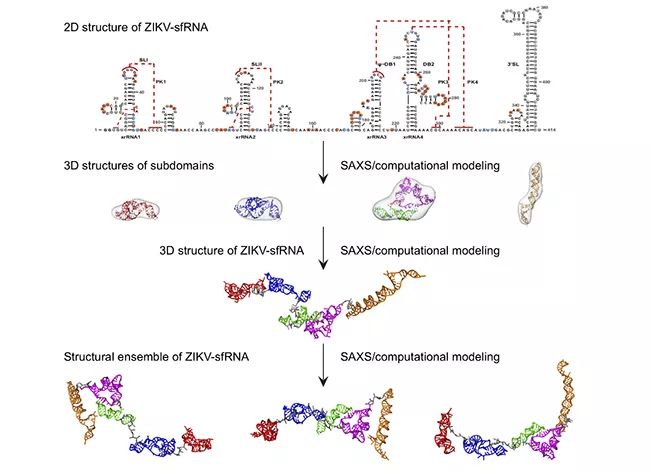
▲ Flow chart of studying the structure of flavivirus sfRNA solution by SAXS and computational simulation.
Long non-coding RNA (lncRNA) is a non-coding RNA with a length of more than 200 nucleotides, which plays a variety of important functions in organisms and is a hot spot in life science research. Like its host, almost all viruses can encode long-chain noncoding RNA. Many mosquito-borne flavivirus, such as dengue virus, Zika virus and West Nile virus, are pathogens that pose a great threat to human health. A large number of long-chain non-coding subgenomic RNA(sfRNA) will accumulate in the infected host cells, which play an important role in the pathogenicity of the virus and the immune escape from the host.
It is of great significance to study the structure-function of long-chain noncoding RNA, but at present, there is no effective means to study its three-dimensional spatial structure, so the three-dimensional spatial structure information of long-chain noncoding RNA is still very little. The length of long-chain non-coding subgenomic RNA from mosquito-borne flavivirus is between 400 and 500 nucleotides, so it is very difficult to study its structure by using traditional structural biology methods, such as X-ray crystallography, nuclear magnetic resonance or cryoelectron microscopy. Fang Xianyang’s research team and their collaborators, combined with small-angle X-ray scattering technology and computational simulation, studied the solution structure and flexibility of long-chain non-coding subgenomic RNA from dengue virus type 2, Zika virus and West Nile virus by step-by-step solution. It is found that the long non-coding subgenomic RNA of flavivirus has multiple subdomains, which have an extended three-dimensional spatial structure in solution, modular structural characteristics similar to multi-domain proteins and great flexibility.
It is particularly interesting that the three-dimensional spatial shapes and structures of the dumbbell-shaped domains in series of sfRNA of Dengue virus, Zika virus and West Nile virus based on small-angle X-ray scattering data are significantly different, which is presumed to be caused by the topological differences of their pseudo-knots in series. This inference is confirmed by further computational simulation, in vitro biochemistry and in vivo cell experiments. This study is an active exploration of the three-dimensional structure of long-chain noncoding RNA. The research results of this paper not only provide important structural information for understanding the physiological function of sfRNA, but also show that the integrated structural biology method based on small-angle X-ray scattering and computational simulation is a feasible method for studying the three-dimensional structure of other long-chain noncoding RNA solutions.
Fang Xianyang, researcher of Tsinghua University Life College, and Qin Chengfeng, researcher of Institute of Microbial Epidemiology, Institute of Military Medicine, Academy of Military Sciences, are co-authors of this article. Zhang Yupeng, a 2013 direct doctoral student of Tsinghua University Life College, Zhang Yikan, a 2016 general doctoral student, Dr. Liu Zhongyu, an associate professor of Institute of Microbial Epidemiology, and Cheng Mengli, a master student of Institute of Microbial Epidemiology, are co-authors of this article.
Paper link:
https://doi.org/10.15252/embr.201847016

Zhu Junming and Xue Lan of Tsinghua University of Public Administration jointly published a document to reveal the impact of China’s carbon emission trading policy on low-carbon innovation.
On September 9th, Associate Professor Zhu Junming and Professor Xue Lan from School of Public Administration of Tsinghua University published a research paper entitled "Low-carbon innovation induced by emissions trading in China" online in the international academic journal Nature Communications, and evaluated the effect of the pilot policy of carbon emissions trading in China on promoting enterprise innovation and its mechanism.
Carbon emission trading is a widely concerned market-based carbon emission reduction policy, which has been adopted by many countries and regions in the world and can play an important role in mitigating climate change. At present, the empirical knowledge about the effect of carbon emissions trading policy mainly comes from the study of EU carbon market. However, people still have little understanding of the scope of this market-oriented policy, the influence of interaction with other policies and the changes in the mechanism brought about by policy design, which limits the further promotion and greater role of the policy.
In order to solve this problem, Associate Professor Zhu Junming and Professor Xue Lan from School of Public Administration of Tsinghua University pay close attention to the pilot policy of carbon emission trading in China since 2013, and use enterprise-level data and patent application information to empirically test the influence and potential mechanism of the policy on low-carbon innovation of enterprises by using the methods of matching and double difference. Low-carbon innovation is helpful to get rid of the path dependence on carbon-intensive economy and energy infrastructure, and alleviate the negative impact caused by the uncertain trend of carbon emission reduction, which is very important to achieve stable emission reduction.
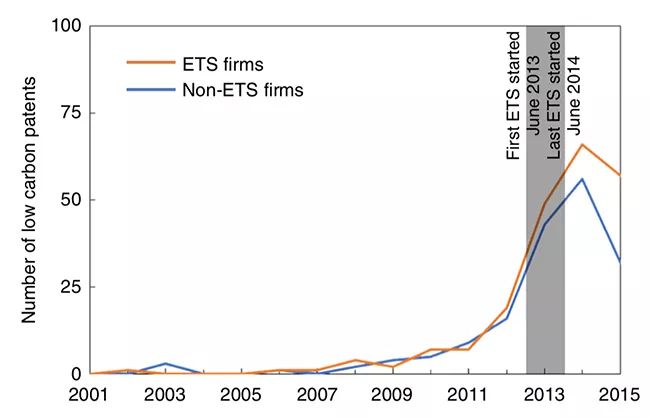
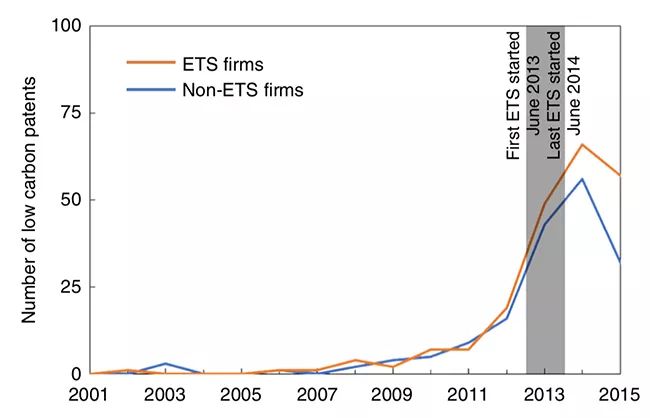
▲ Patent application trends of carbon trading enterprises and non-carbon trading enterprises.
It is found that, on the basis of the impact of policies such as energy conservation and emission reduction and low-carbon pilot projects, carbon emission trading has promoted trading enterprises to increase 1.75 low-carbon patent applications within two years, which is close to the policy effect of two low-carbon patent applications in the EU carbon market within five years, and at the same time, it has increased the applications of other types of patents, especially other green technology patents. The influence scope of the policy goes beyond the carbon emission trading enterprises, and the influence on enterprises that have not been included in carbon trading but may be included in the future is also very significant. It is also found that the innovation behavior of enterprises is not affected by the price of carbon trading, but by the way of quota allocation. This series of research findings provide a reference for the construction of China’s national carbon market.
Associate Professor Zhu Junming from the School of Public Administration of Tsinghua University is the first author and co-author of the article, while Deng Xinghua, a former postdoctoral fellow in Tsinghua University and now a lecturer at Southwestern University of Finance and Economics, is the co-author of the article, and Professor Xue Lan and Fan Yichun, an undergraduate graduate of Tsinghua University and now a master of Massachusetts Institute of Technology, are the co-authors of the paper.
Paper link:
https://www.nature.com/articles/s41467-019-12213-6

Zhong Yi’s research group of Tsinghua Institute of Life reveals the downstream molecular mechanism of active forgetting of different memory components.
On September 5th, the research group of Zhong Yi, School of Life Sciences, Tsinghua University published a research paper entitled "Genetic Dissection of Active Forgetting in Labile and Consolidated Memories in Drosophila" in PNAS.
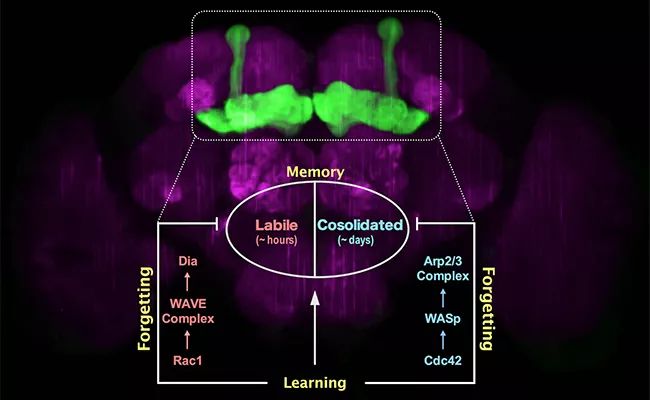
In this paper, the downstream molecular mechanisms of active forgetting of unstable short memory and stable long memory in Drosophila were systematically compared and analyzed for the first time. It was found that the active forgetting of unstable short memory was mediated by Rac1/WAVE complex/ Dia pathway, while the active forgetting of stable long memory was mediated by Cdc42/WASp/ Arp2/3 complex pathway. As Dia and Arp2/3 complex are reported to regulate the linear growth and branching growth of neurofilament skeleton respectively, the research results of this paper provide an interesting possibility: the active forgetting of memory components with different stability may be related to the growth mode of different forms of neurofilament structure.
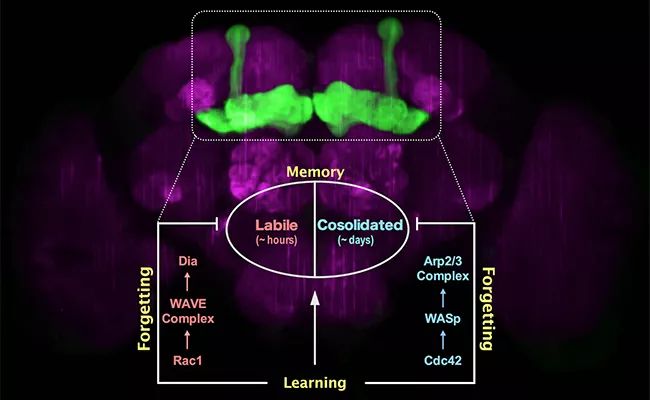
▲ Analysis of forgetting mechanism of stable memory and unstable memory.
As early as 1885, Hermann Ebbinghaus, a German psychologist, described the forgetting curve of the human brain for newly acquired memories, and began the journey of human beings to explore the mystery of forgetting. In the following 100 years, many scholars tried to find the mechanism of forgetting by using the method of experimental psychology, and also created a variety of theories to explain the phenomenon of forgetting, but ultimately failed to get a unified answer. As a basic feature of human memory, forgetfulness has been widely studied and reported in psychology, but almost no one cares about it in the research of neurobiology, and it has become a "forgotten corner". The main reason may be that the forgetting process is simply regarded as another description of memory failure, and it is a passive process (if you remember poorly, you will forget quickly, and if you remember well, you will forget slowly), thus ignoring the unique molecular mechanism of forgetting itself.
Until 2010, Professor Zhong Yi’s research group discovered the biological mechanism of active forgetting for the first time, that is, the learning process itself will activate the molecular pathway of forgetting signals to actively forget the formed memories. This active forgetting mechanism does not affect the quality of learning itself, but specifically affects the speed of forgetting acquired memories. Moreover, different memory components will have their own active forgetting mechanisms. For example, in the olfactory punishment learning of Drosophila, one training (5 minutes) can produce two completely different memory components at the same time: unstable anesthesia-sensitive memory (ASM) and relatively stable anesthesia-resistant memory (ARM) which lasts for more than one day. Zhong Yi’s research group has found that a study session can activate Rac1 molecule in the small G protein family to erase the unstable anesthetic sensitive memory that lasts for several hours, and activate Cdc42 molecule, another member of the small G protein family, to forget the more stable anti-anesthetic memory that lasts for more than one day. However, the specific downstream molecular pathways of the forgetting mechanism mediated by Rac1 and Cdc42 are not clear.
Recently, the research paper published online by Zhong Yi’s research group analyzed the downstream molecular pathways of these two forgetting mechanisms for the first time: Rac1/WAVE complex/ Dia pathway mediates the active forgetting of short-term memory components (unstable anesthesia-sensitive memories that last for several hours), while Cdc42/WASp/ Arp2/3 complex pathway mediates the active forgetting of long-term memory components (relatively stable anti-anesthesia memories that last for more than one day). Both of these active forgetting mechanisms occur in the mushroom body of the learning and memory center of Drosophila (the mushroom body contains about 4000 neurons, and the whole brain of Drosophila has more than 100 thousand neurons). Interestingly, cell biology research found that Dia was directly responsible for the linear growth of microfilament skeleton, while Arp2/3 complex was responsible for the branching growth of microfilament skeleton. This suggests that the active forgetting function of different memory components may be related to different forms of dynamic changes in the microfilament skeleton structure of neurons. On the one hand, these research results bring the academic understanding of the molecular mechanism of forgetting into a more detailed dimension, on the other hand, they provide more effective pharmacological targets for directly regulating the function of forgetting.
Gao Yang, a Ph.D. student in the School of Life Sciences of Tsinghua University, Shuai Yichun, a researcher in the Janelia Research Campus of the United States, Zhang Xuchen, a graduate doctoral student in the School of Life Sciences of Tsinghua University, and Peng Yuwei, a doctoral student, are the co-first authors of this paper. Professor Zhong Yi from Tsinghua University and Assistant Researcher Li Gan are co-authors of this article.
Paper link:
https://doi.org/10.1073/pnas.1903763116

Xiao Bailong of Tsinghua Pharmaceutical College and Li Xueming’s research group of School of Life jointly published a paper in Nature.
Reveal the delicate molecular machines structure and mechanism that mediates human tactile perception.
On August 21, Nature (Nature) The journal published the research paper "Structure and Mechanizing of the Mammalian Tactical Channel Piezo2" written by Xiao Bailong’s research group of Tsinghua University Pharmaceutical College and Li Xueming’s research group of College of Life Sciences. The three-dimensional structure and exquisite working mechanism of high-resolution cryoelectron microscope, a mechanical force molecular receptor-PIEZO 2 ion channel, which gives human tactile perception ability, are reported for the first time. This research is another important research achievement in this field after the two research groups reported in Nature on January 22nd, 2018 the high-resolution three-dimensional structure and molecular mechanism of another member of the mechanically gated PIZO channel family-PIZO 1 ion channel, which not only strongly promoted the understanding of the structural basis and molecular mechanism of PIZO channel family, but also laid a solid foundation for drug development based on PIZO channel. At the same time, Nature published a commentator’s article and spoke highly of the research.
Touch, as one of the five senses, not only gives us the ability to perceive pleasant touches such as shaking hands, stroking and kissing to maintain normal social behavior, but also bears the biological basis for human beings to skillfully use various tools such as touch screen, mobile phone or mouse. However, under pathological conditions such as tissue injury or inflammation, abnormal tactile perception function can lead to severe mechanical hypersensitivity (also known as touch pain). For example, patients with cancer or arthritis often suffer from severe pain caused by slight touch such as dressing or walking, which seriously affects individual health and quality of life. In addition, autistic people usually show more sensitive tactile perception than ordinary people. Recent studies suggest that this abnormal tactile function may be one of the important causes of autism.
Tactile perception originates from the response of mechanical force sensing molecular receptor-mechanically gated cation channel expressed on primary sensory neurons to mechanical force stimulation, which causes extracellular cations, such as sodium ions and calcium ions, to flow into cells, which in turn induces nerve cell excitement and signal transmission, and finally leads to tactile sensation. In 2010, the research group of Professor Ardem Patapoutian of Scripps Institute in the United States identified that the Piezo gene family encodes the necessary components of mechanically gated cation channels in mammals. In 2012, Dr. Xiao Bailong, who is engaged in postdoctoral research in Professor Patapoutian’s research group, and his colleagues reported in the journal Nature that Piezo protein constitutes the core pore component of mechanically gated cation channels, thus establishing a brand-new ion channel family type of mechanically gated Piezo channels for the first time.
Subsequent studies have proved that Piezo2 mediates the mechanical perception of tactile, proprioception (such as posture balance perception) and visceral perception (such as lung contraction and expansion, blood pressure perception and heart rate regulation) in mammals, while Piezo1 has been found to act as a molecular receptor of mechanical force in a variety of cell tissues and participate in regulating the development of blood vessels and lymphatic vessels, blood pressure homeostasis, bone formation and remodeling and many other functions. The genetic mutation of Piezo gene has been found to cause many human genetic diseases, including erythrocytopenia syndrome, lymphatic edema, distal joint contracture, tactile loss and so on. The human body with the loss-of-function mutation of Piezo2 not only shows tactile and proprioception defects, but also loses the perception of mechanical hypersensitivity in pathological state, which confirms that Piezo2 channel can be used as an important target for developing new analgesic drugs.
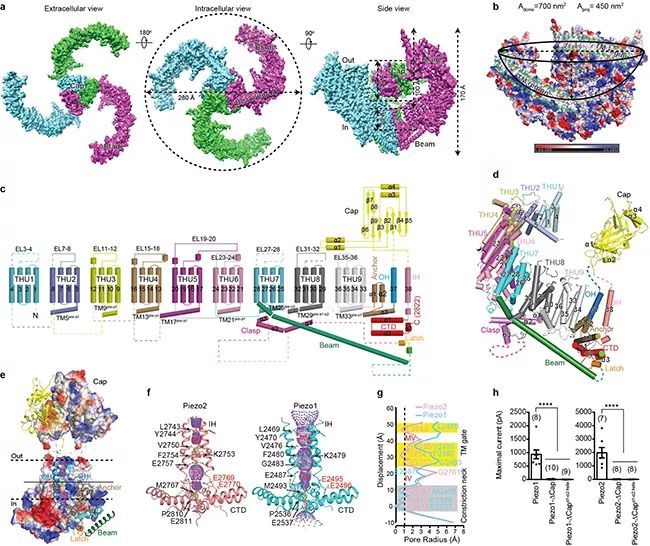
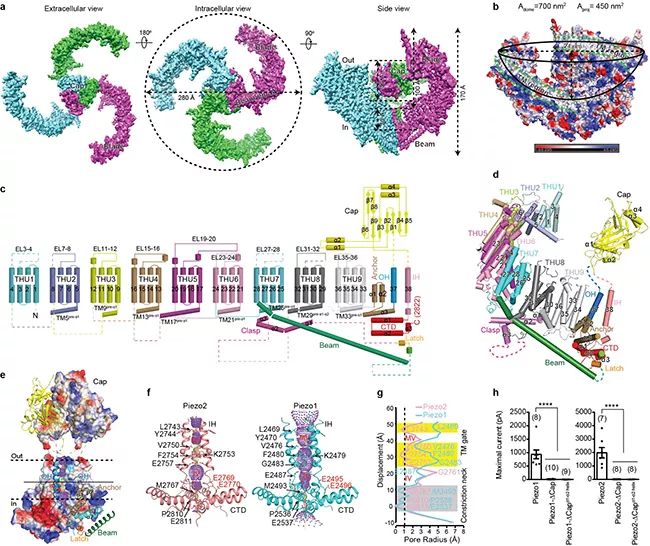
▲ Figure A, schematic diagram of frozen electron microscope of Piezo2, in which each subunit is represented by a different color; B, a schematic diagram of a dome-shaped structure surrounded by three outward twisted transmembrane paddle areas, and the transmembrane area is marked by the green dotted line; C, schematic diagram of the topological structure of Piezo2 with 38 transmembrane regions; D, a subunit structure of Piezo2 constitutes a display diagram; E, Piezo2 central pore module area; F, the central pore area of Piezo2 and Piezo1 surrounded by IH-CTD; The radius distribution diagram of the central pore area of G, Piezo2 and Piezo1, including the switch gate of transmembrane area and the Constriction neck); of intracellular area; H, Piezo1 and Piezo2 mutants with deletion of hat region lack the current induced by mechanical stimulation.
Dr. Xiao Bailong’s research group comprehensively uses the multi-disciplinary research methods, such as biochemical structure, electrophysiological patch clamp, Qualcomm dosage drug screening, transgenic mouse model and human genetics, and focuses on solving the key scientific problems of how the mechanically gated Piezo channel converts mechanical stimulation into electrochemical signals, and how it uses its own mechanical sensitivity and channel characteristics to determine related physiological and pathological functions, and is committed to developing new drugs and technologies targeting Piezo channel. Up to now, as a correspondent (including co-author), he has made a series of important research achievements in the three-dimensional structure analysis, molecular mechanism revelation, small molecule drug discovery and physiological and pathological function exploration of Piezo channel.
In this latest Nature paper, relying on the technical system and research platform established by two research groups for analyzing the structure of Piezo1 channel by cryo-electron microscope, the researchers have made unremitting efforts for more than six years to overcome the difficulty of extremely low expression of mouse-derived Piezo2 with a total length of 2,822 amino acids. Through continuous exploration and optimization of protein purification conditions and sample preparation methods by cryo-electron microscope, a stable and homogeneous protein sample was finally obtained for data collection by cryo-electron microscope. In the process of structural analysis, Piezo protein is extremely flexible, which brings great challenges to the resolution of high-resolution structures. The researchers expanded the symmetry of Piezo2 data particles, cut them into three parts, and calculated them independently, so as to obtain high-resolution structures of each part and then put them together into a complete structure, overcoming the flexibility problem, and finally obtained a three-dimensional structure with an overall resolution of 3.6-3.8 angstroms (Figure A), and successfully analyzed the complete topological structure of Piezo2 protein including 38 transmembrane helical regions (Figures C and D).
The researchers found that the overall three-dimensional structure of Piezo2 channel is similar to that of Piezo1 channel previously analyzed, and it is assembled into a trilobal propeller-like structure with 114 transmembrane helical regions in the form of homotrimer (Figure a-d), and it is established that Piezo channel is a kind of large membrane protein with the most transmembrane times so far. Among them, the 1st to 36th transmembrane helix regions (TM1-36) are sequentially assembled into 9 repetitive structural units with the 4th transmembrane helix region as the unit, and this characteristic structural unit was previously named THU (Transmembrane Helical Unit) with the abbreviation of Tsinghua University (Figure c, d). Very interestingly, these nine thus are connected in series to form a special non-planar transmembrane "Blade" structure, which leads to the three blades enclosing into a dome-shaped structure with a diameter of 28nm and a depth of 10nm (Figure B). Researchers speculate that this dome-shaped PIZO protein-cell membrane system may be one of the structural foundations of PIZO channel with high mechanical sensitivity.
The extracellular "Cap" domain located above the central pore region is completely embedded in the bottom of the dome (Figures A and B), while there is a 9nm long Beam structure inside the cell, which connects the peripheral end of the blade to the intracellular position of the central pore region (Figures a-e). The 38th transmembrane helix (named inner helix, IH) and the trailing intracellular carboxyl terminal domain (CTD) form a central pore part (Figures E and F), including a narrow neck located in the transmembrane region and cytoplasmic region (Figures F and G), suggesting that the Piezo channel structure is in a closed state. The structural comparison between Piezo2 and Piezo1 shows that the transmembrane region narrow site is completely closed in Piezo2 structure, but open in Piezo1 (Figure f, g). Based on this, the researcher proposed that the narrow site forms a switch gate of transmembrane region (Figure f, g). Further structural analysis and electrophysiological function experiments show that the gate may be controlled by the rotation of the top hat domain (Figure H). However, because the narrow constriction neck located in the cytoplasm region is in the closed state in both Piezo1 and Piezo2 structures (Figure f, g), it remains to be further proved whether this site is controlled by the conformational changes of the blade and long rod structure as another switch gate.
Dr. Xiao Bailong said that through the full cooperation with Dr. Li Xueming’s research group, the analysis of the structure from Piezo1 to Piezo2 not only strongly promoted the understanding of the structure and mechanism of mechanical gating of Piezo channel in mammals, but also provided a solid foundation for exploring the mechanism of human diseases caused by the dysfunction of Piezo channel and the identification and discovery of related drugs. Relying on the all-round research platform and academic accumulation of Piezo channel established by their research group, they will devote themselves to the drug discovery and development of Piezo channel.
Dr. Xiao Bailong from Tsinghua University College of Pharmacy and Dr. Li Xueming from College of Life Sciences are co-authors of this paper. Wang Li, a postdoctoral fellow at Tsinghua University College of Pharmacy, Zhou Heng, a 2015 doctoral student at School of Life, Zhang Mingmin, a 2013 doctoral student, and Liu Wenhao, a 2016 doctoral student, are the first authors. In addition, Dr. Deng Tuan, Zhao Qiancheng (now a postdoctoral fellow at Yale University) and Li Yiran of Xiao Bailong’s research group also participated in some research work. Dr lei Jianlin of Tsinghua University cryo-electron microscope platform provided help for data collection of cryo-electron microscope.
Paper link:
https://www.nature.com/articles/s41586-019-1505-8.pdf
Graphic | School of Medicine, School of Life, Department of Electronics, School of Public Administration, School of Pharmacy
Editor | Li Jing